Covid 19 Antibody Eua - Covid-19 Realtime Info
Press release globenewswire.

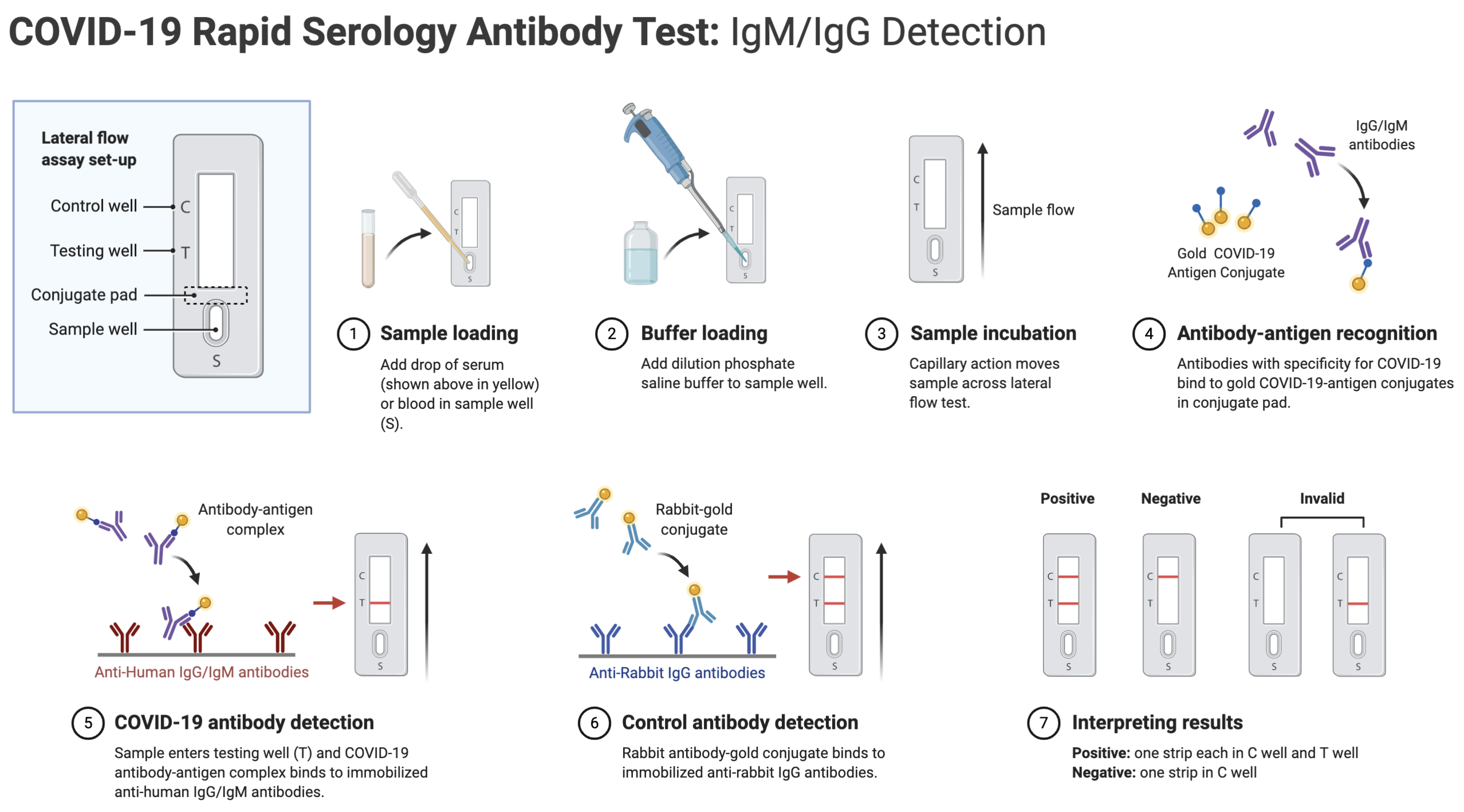
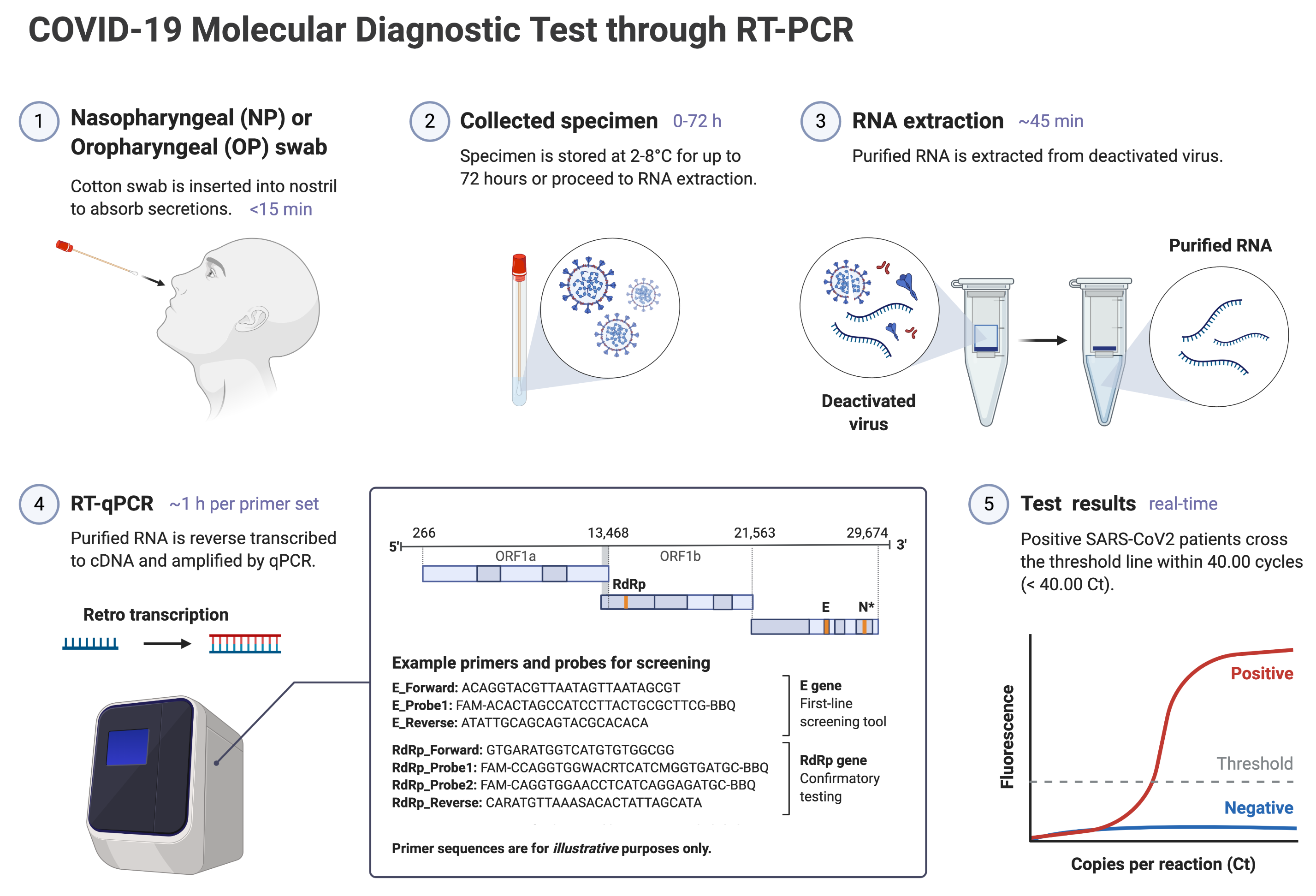
Covid 19 antibody eua. 20 2020 0431 pm. Chembio dpp covid 19 igmigg system a sars cov 2 antibody test. Fda authorized eua covid 19 antibody tests. Schedule an appointment for nasal swab for covid 19 testing today.
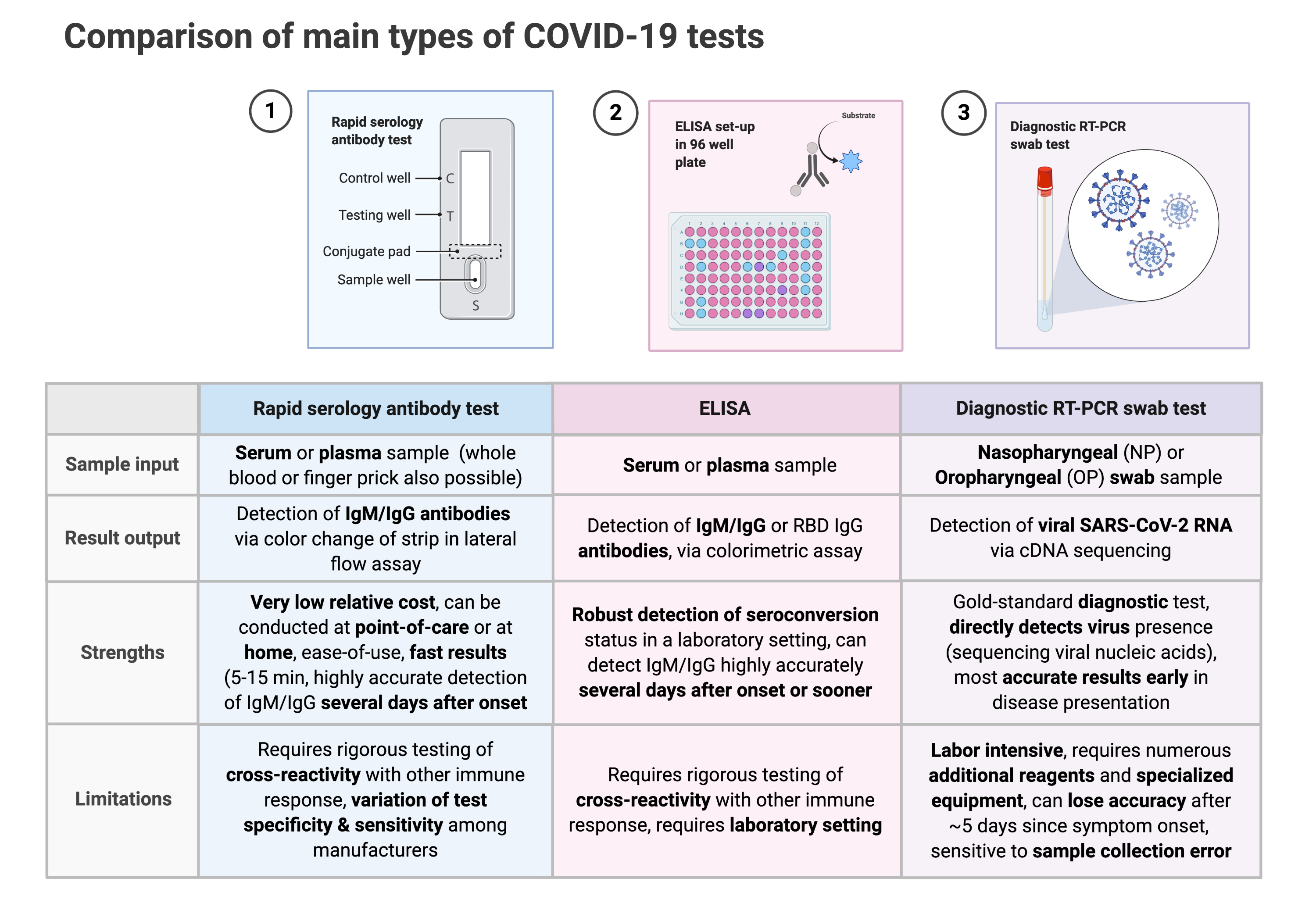
Trinity biotech announces eua submission for covid 19 elisa antibody test. In vitro diagnostics euas for covid 19 tests. Food and drug administration revoked the emergency use authorization eua of the chembio diagnostic system inc. The igm test is typically used together with the igg test for a.
Antibodies are produced in the blood to combat infections and viruses like the coronavirus. Diazyme continues to serve during the public health emergency with innovative products in covid 19 serology antibody testing. Click to complete covid 19 forms online. Government health officials had urged fda not to issue an eua on plasma for covid 19 which led trump to post a tweet accusing them of being part of the deep.
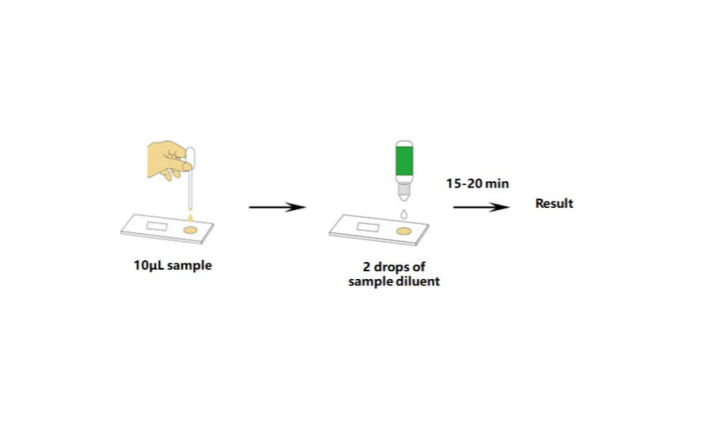
In continuing response to the covid 19 pandemic on march 24 2020 and based on the february 4 2020 hhs eua determination the hhs secretary declared that circumstances exist justifying the. Serology tests detect the presence of antibodies in the blood when the body is responding to a specific infection like covid 19. Over the past week several us. In vitro diagnostic ivd devices are tests performed on samples taken from the human body such as swabs of mucus from inside the nose or back of the.